With FastFinder 3.0, we've taken another step towards full PCR automation. In this blogpost, you'll find a list of the most important features and changes and how they will impact your laboratory.
Our new development efforts include a QC module that can be set up to automatically track control samples, apply Westgard rules and plot Levey-Jennings charts.
We've also implemented a LIMS export functionality. This export can be configured to generate standard ASTM & HL7 exports, but can also be customized to whatever your LIMS requires.
Furthermore, we've implemented a two-step validation functionality, which will smoothen your QA efforts by automating the audit trail & save time by proposing a more focused review screen.
Lastly, we've added three new PCR platforms and a lot of small functionality, usability & performance issues were solved.
Quality control module
The bulk of our development efforts went into the QC module. This brand new module will allow you to say goodbye to your spreadsheets, macros or external QC follow-up software.
We've built this module by involving important stakeholders into the development process. We repeatedly asked what the needs were and started creating a comprehensive list of requirements. If you're interested in how we continuously improve our product based on customer feedback, read about our ISO 13485 certificate and lean QMS here.
First, you set up the Westgard rules applicable for your laboratory and choose the controls and device that need to be tracked. You can choose to manually input a mean (some commercial assays require this option) or have a mean calculated by applying floating statistics over any amount of runs you prefer.
In any subsequent analysis, QC rules are automatically calculated during the run. If a violation occurs, the user will be warned and asked to describe why he chooses to approve/ reject the results. If a sample violates one of QC rules, the laboratory can opt to reject all samples linked to the control sample. This way, none of the corrupted results will reach the LIMS.
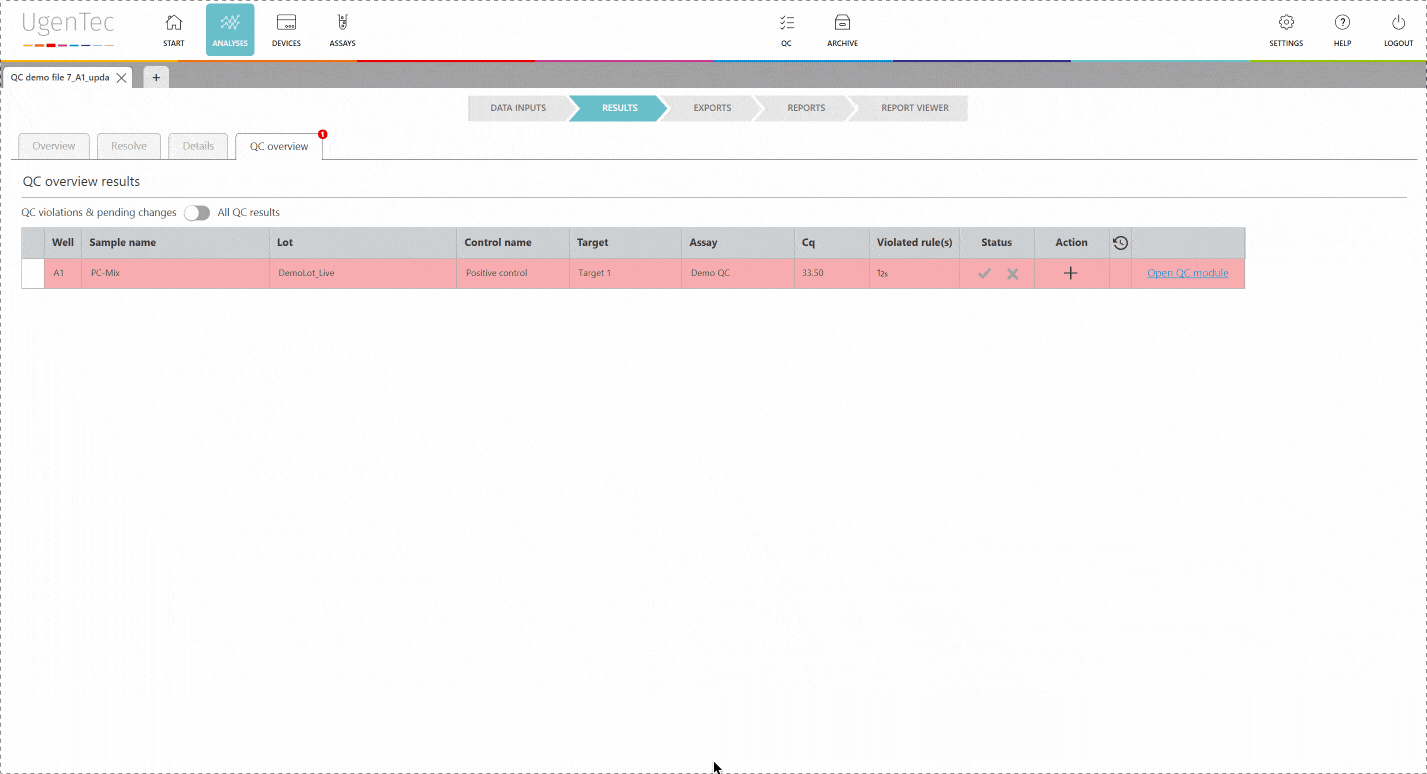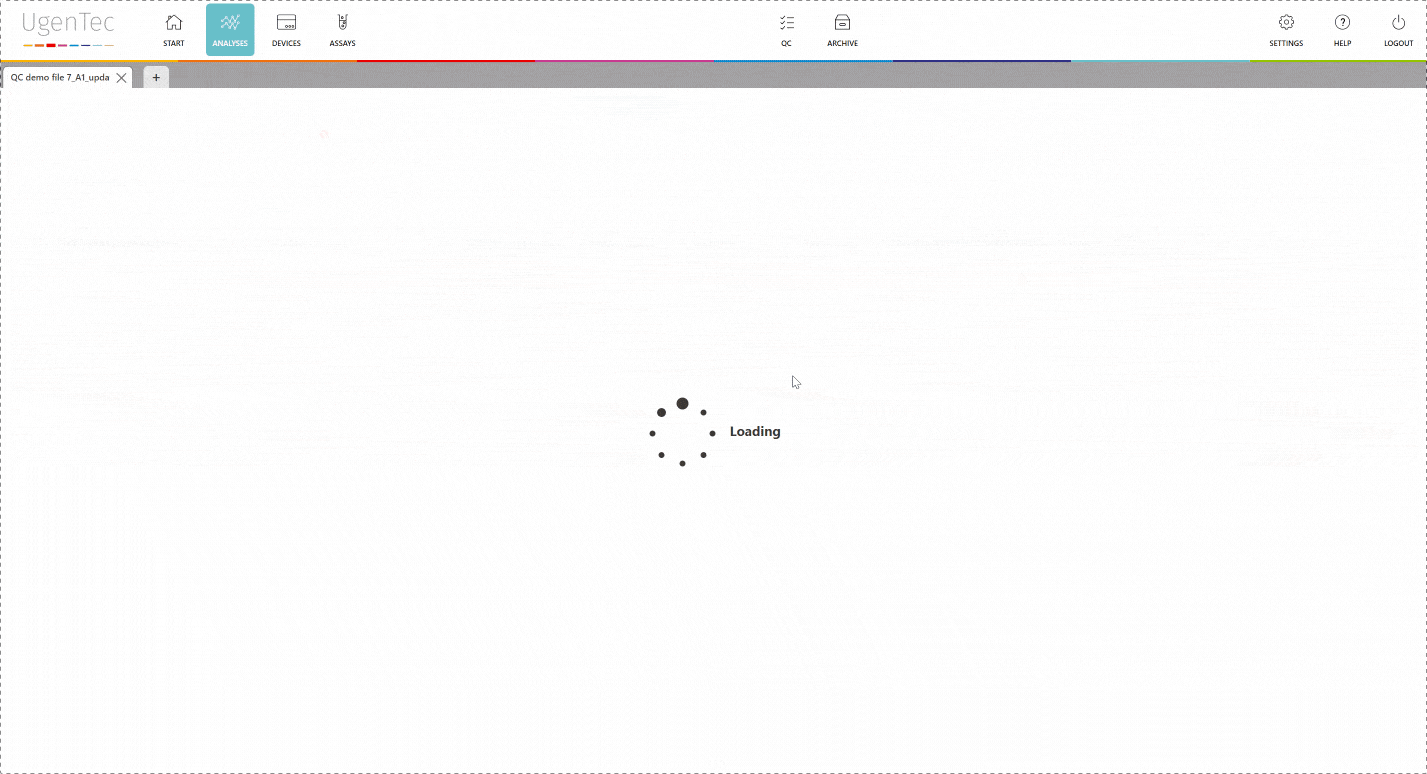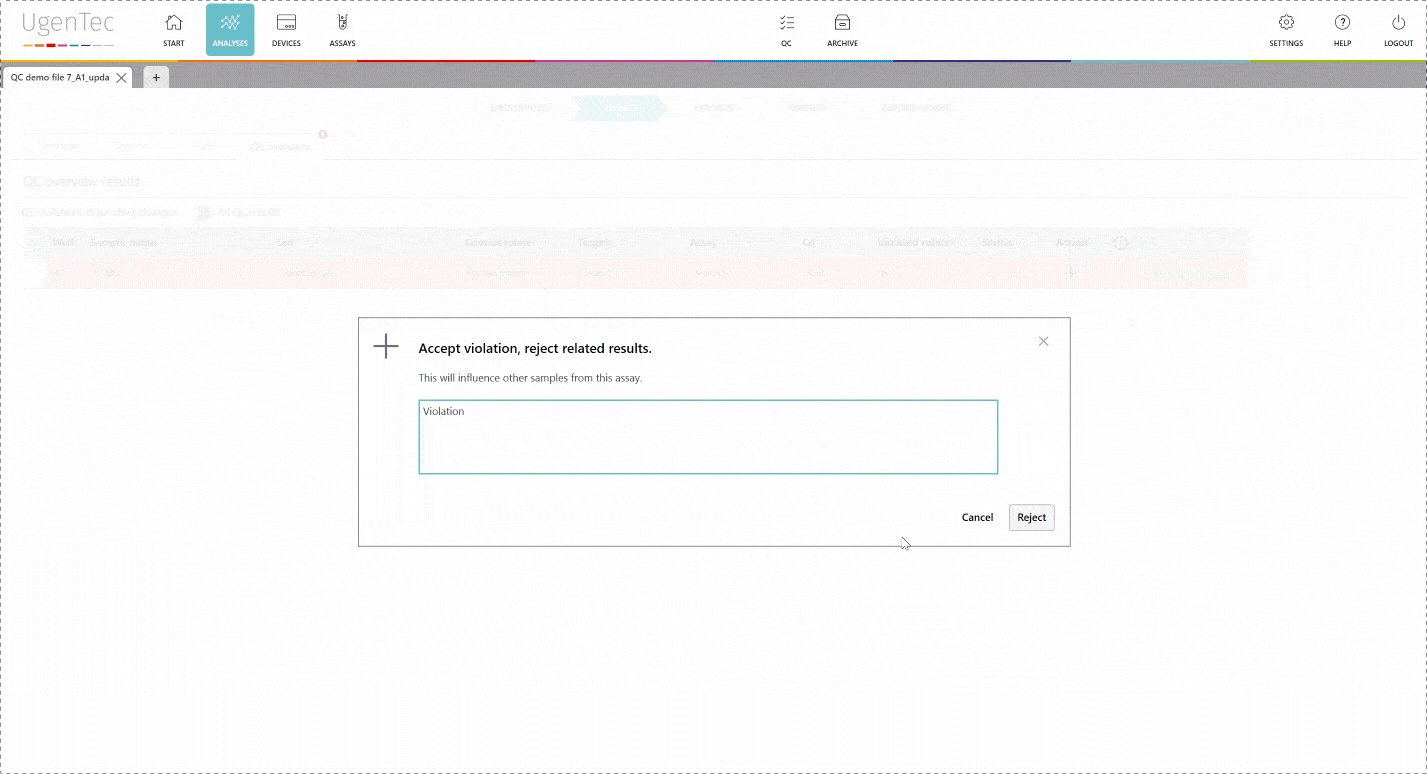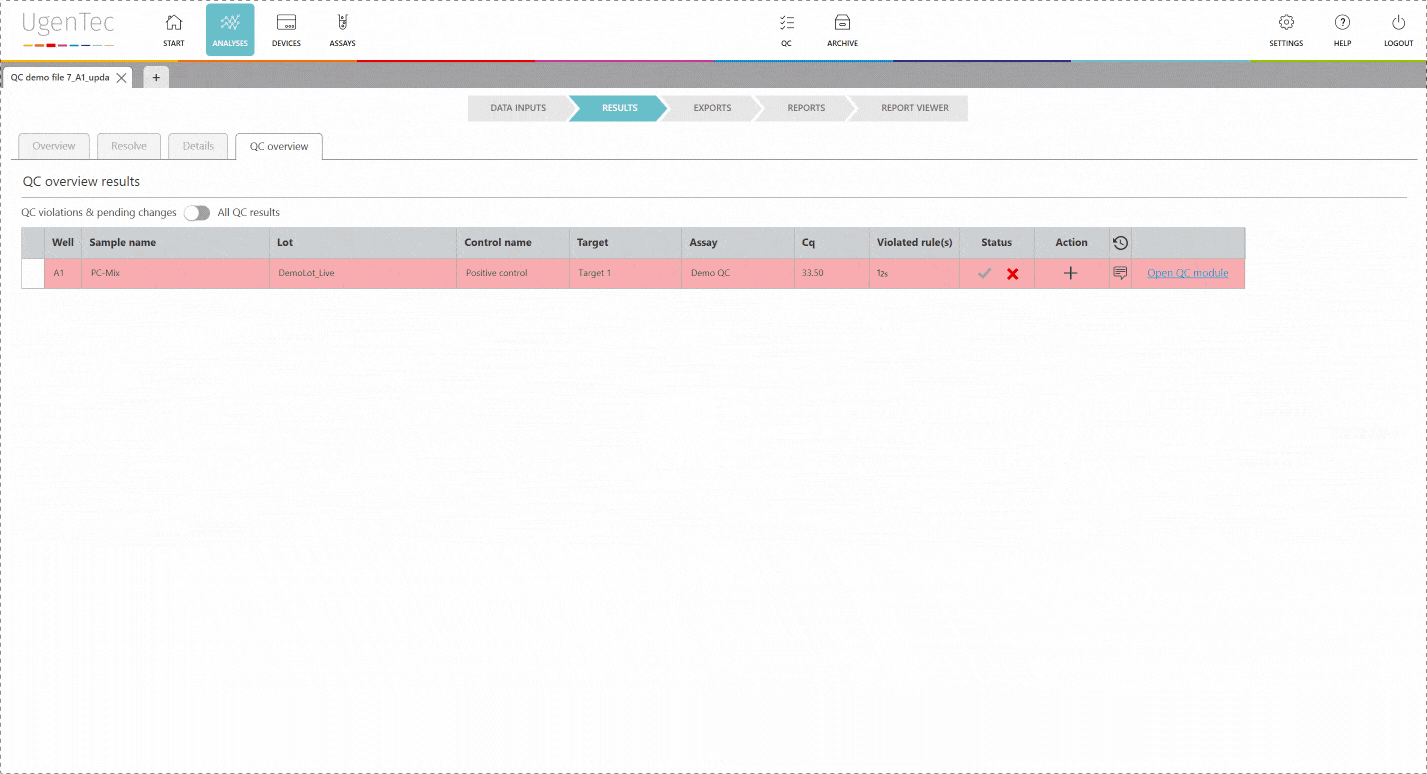
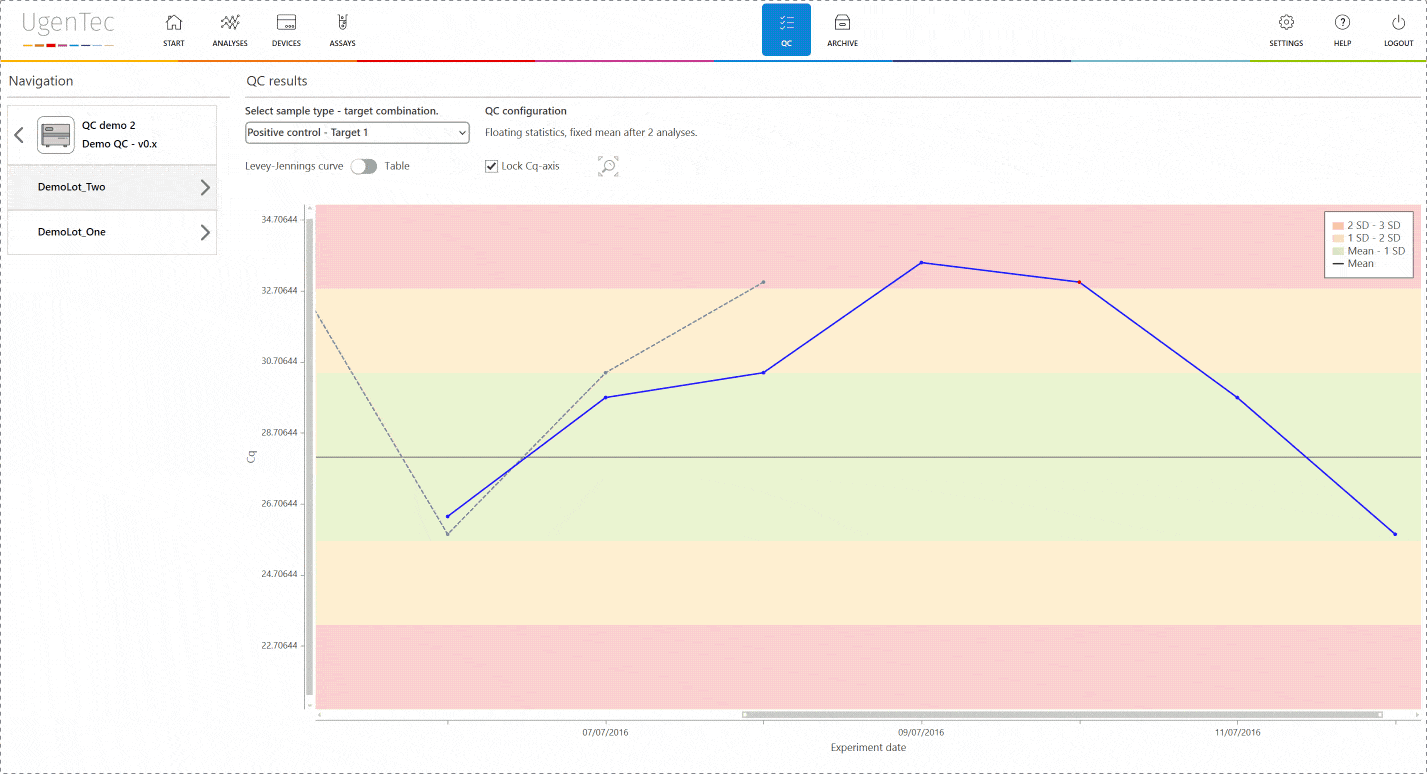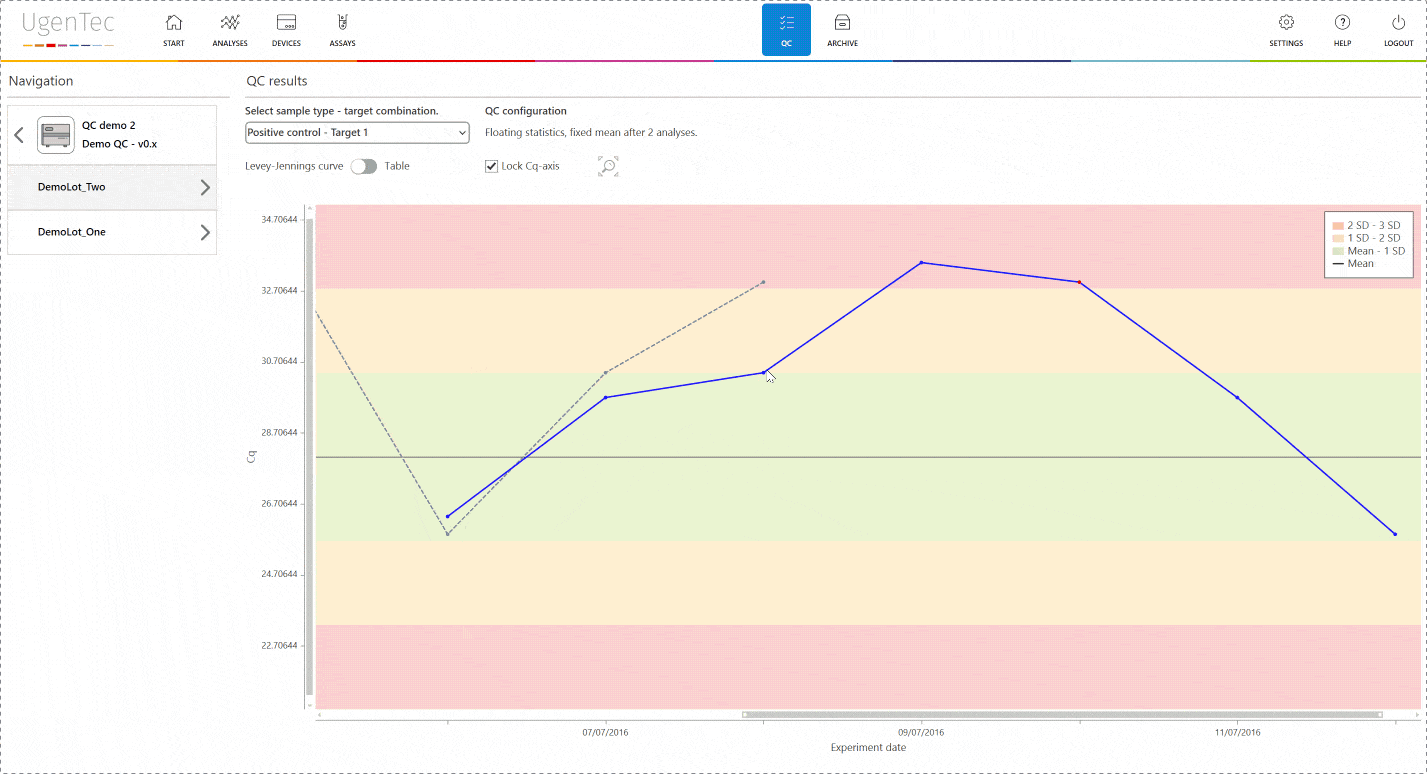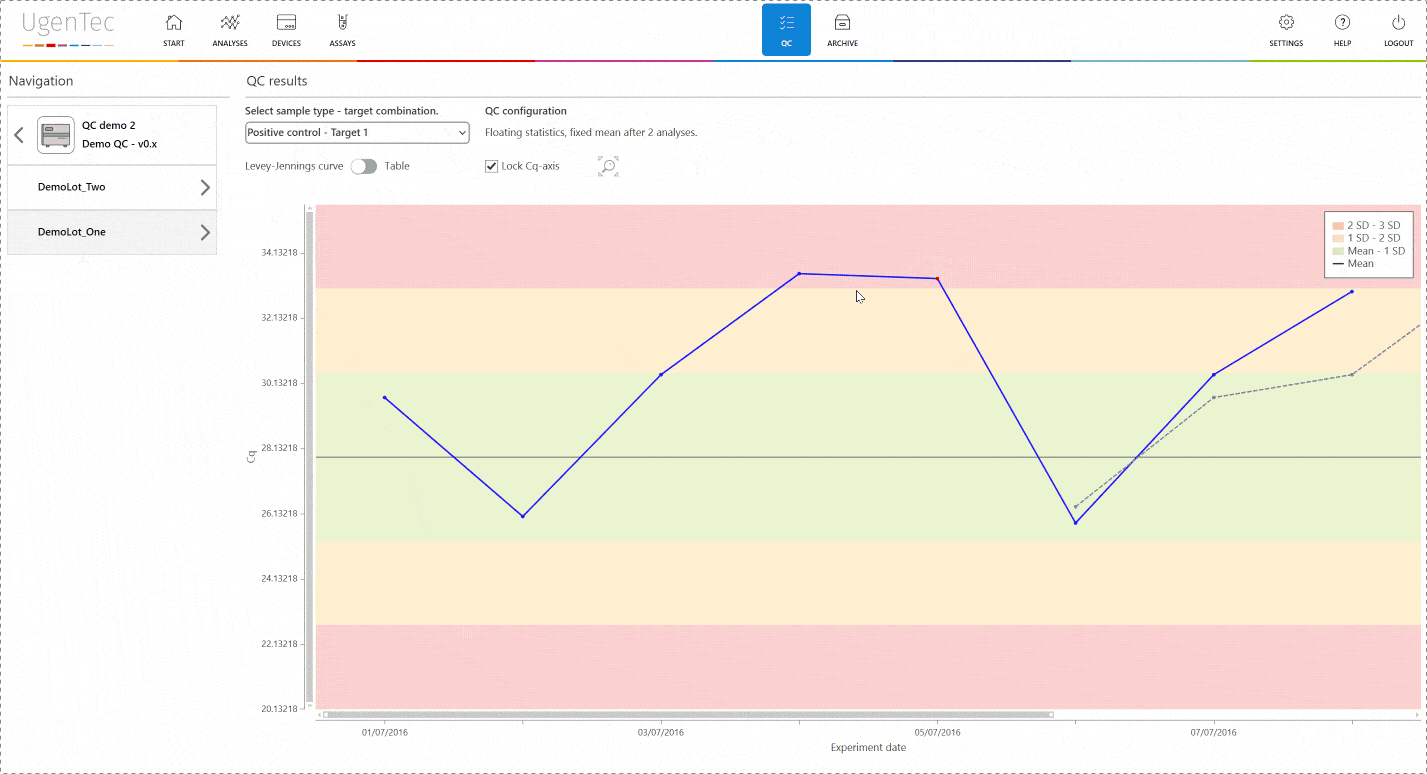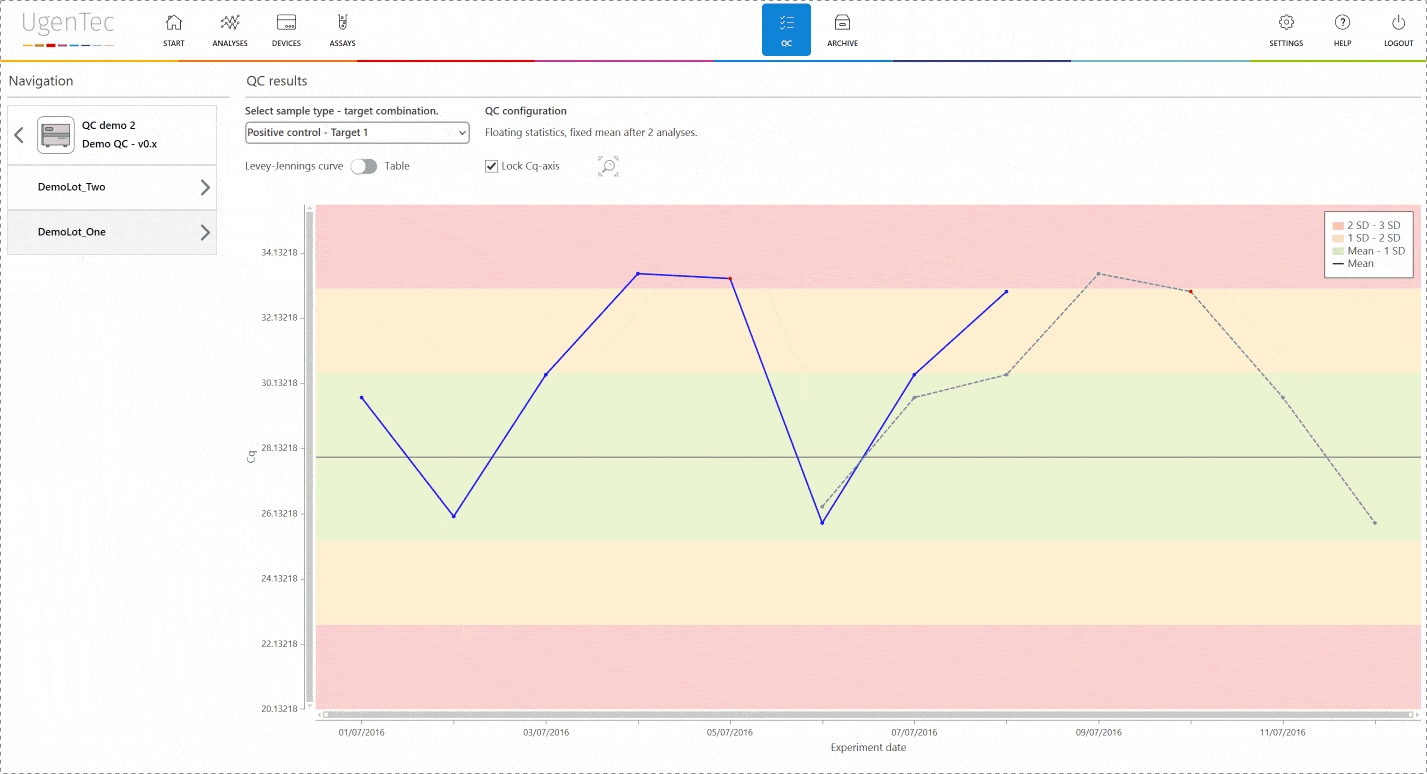
If a scientist wants to get a better grasp of exactly how extreme the violation is, he can go to the QC module with a single click to see how exactly the deviation is positioned in the context of other control measurements.
You can also compare multiple lots to one another when a certain lot is displaying an unusual trend or shift. This way, parallel batch control can be performed. All QC efforts are digitally audit trailed and can be consulted at any time.
LIMS Export
Transferring interpreted results from your spreadsheet, software or head into the LIMS has always been a pain. From a QA perspective, it's the perfect occasion to let typing errors slip into an otherwise pretty secure workflow.
FastFinder can now export files that are compatible with the most common LIMS protocols (ASTM & HL7). We offer a standard integration, which should do the job in most cases, but can customize the connection based on your requirements.
Two-step validation
Implementing a two-step validation procedure is a good way to ensure only qualitative results leave your laboratory. However, a lot of paperwork and complicated procedures are often involved in implementing a two-step strategy.
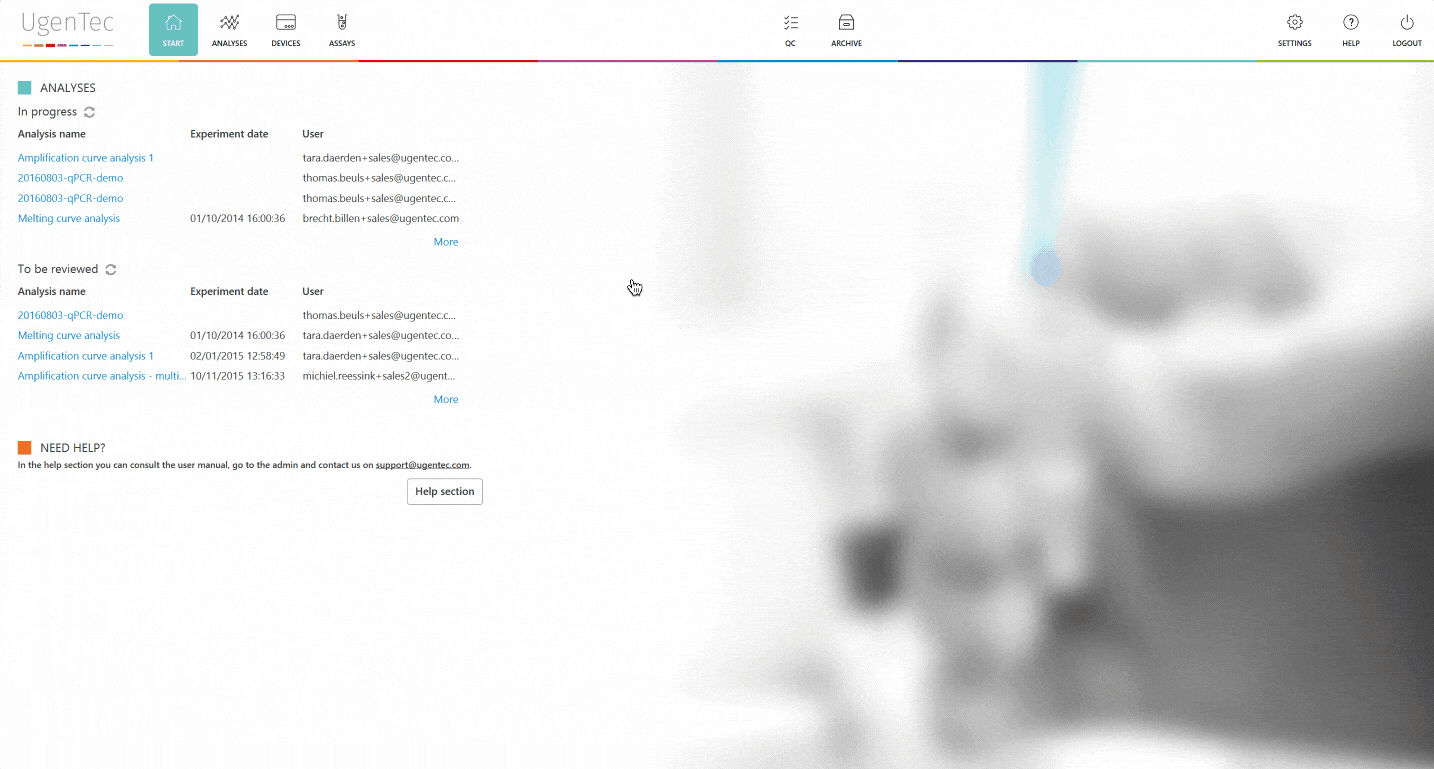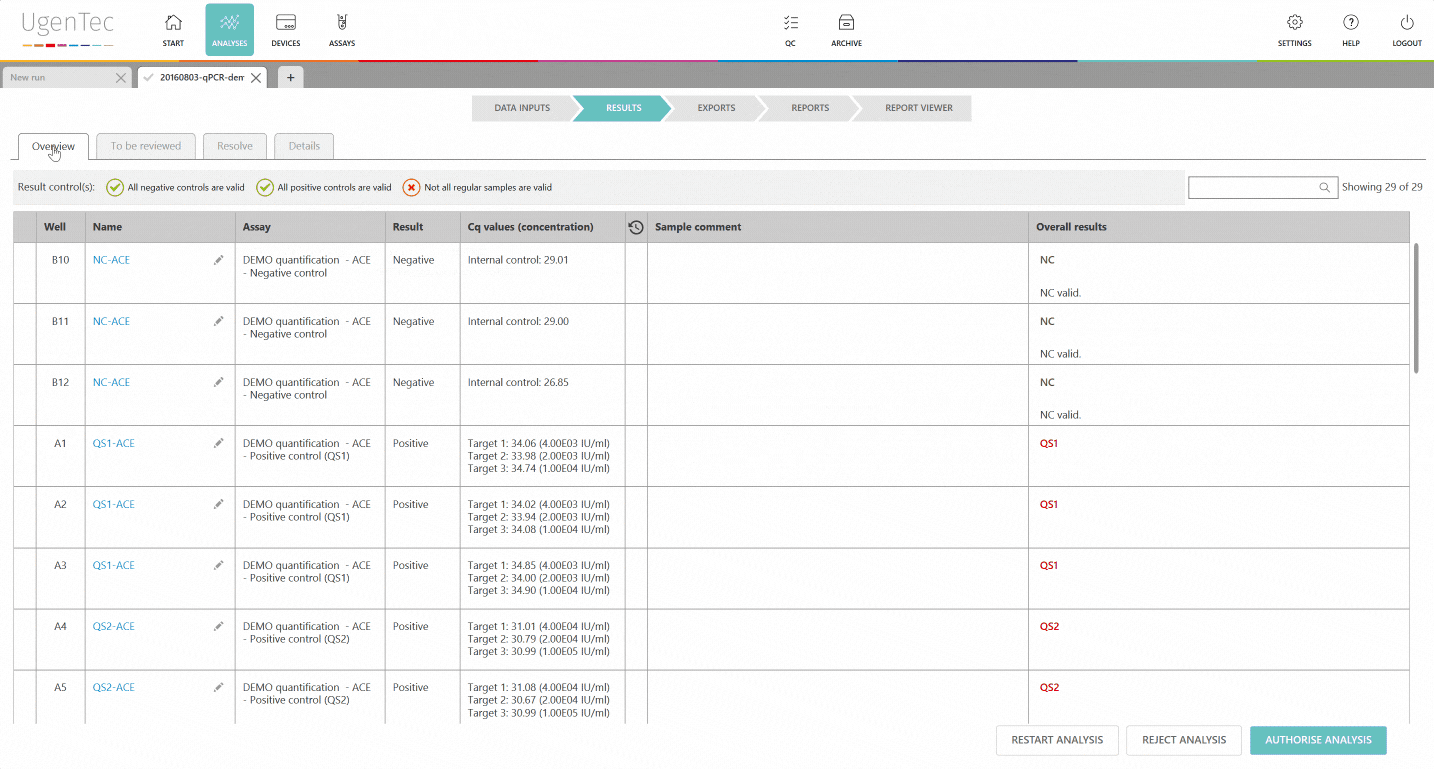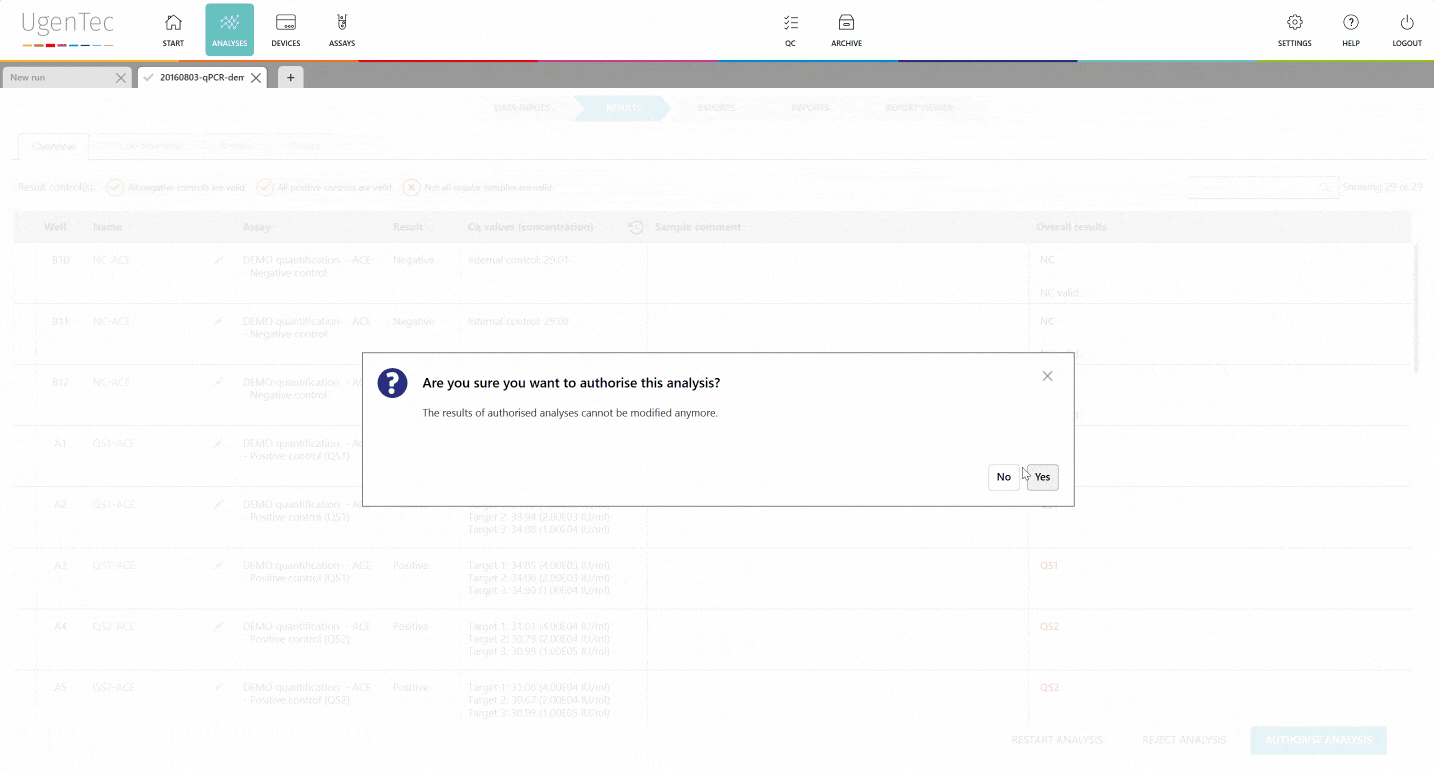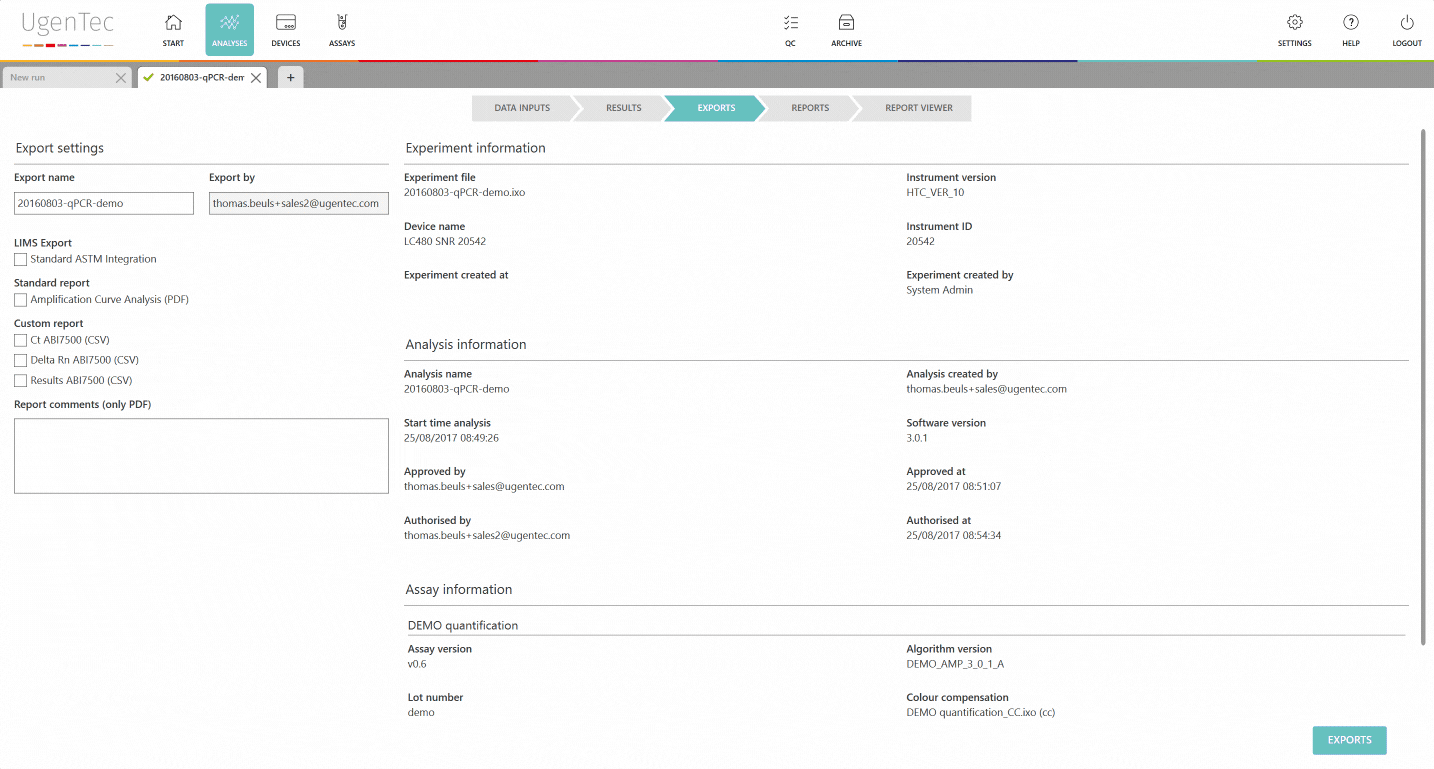
By enabling two-step validation in FastFinder, everything is done automatically for you. An audit trail is logged to each unique login and second reviewers automatically show the results that require attention.
The first reviewer approves the results and is automatically exempt from authorizing the results.
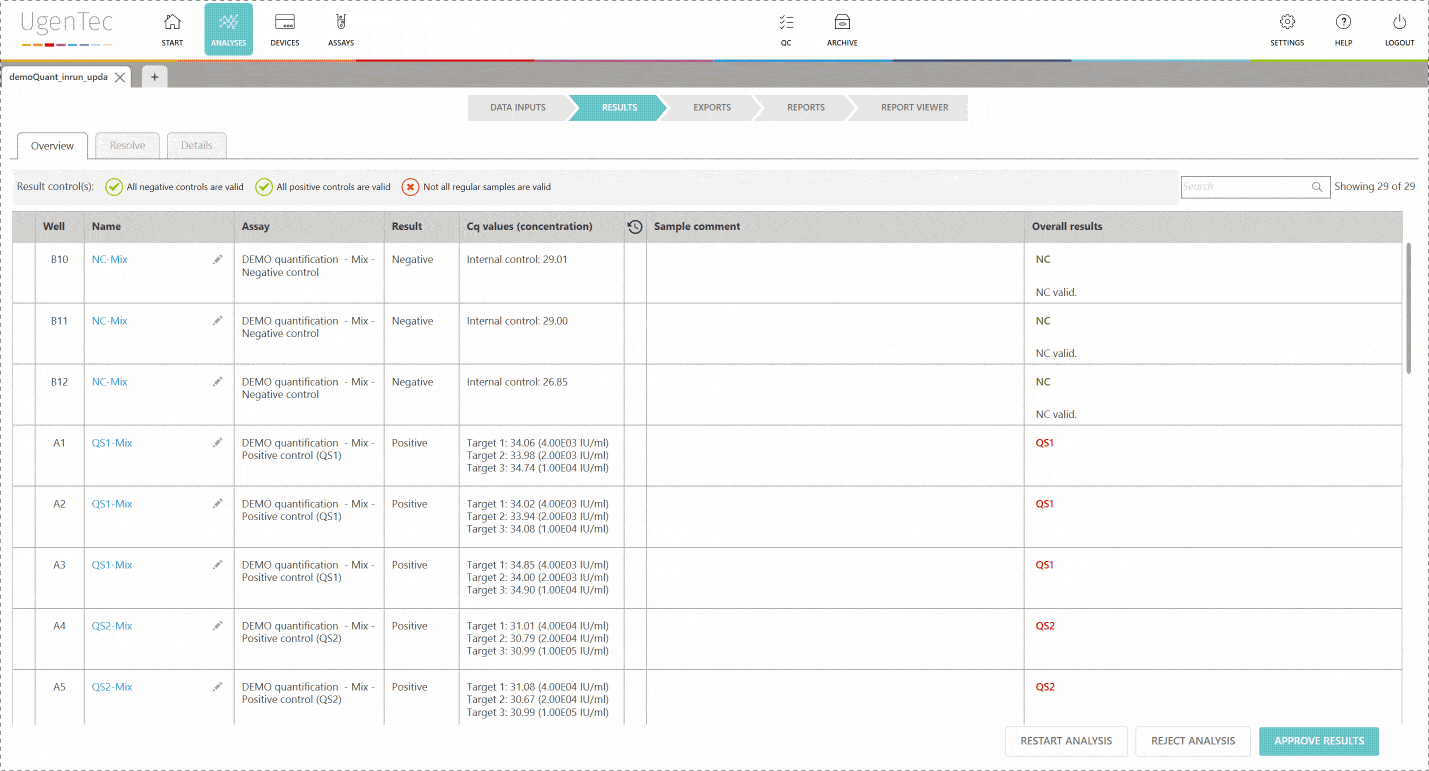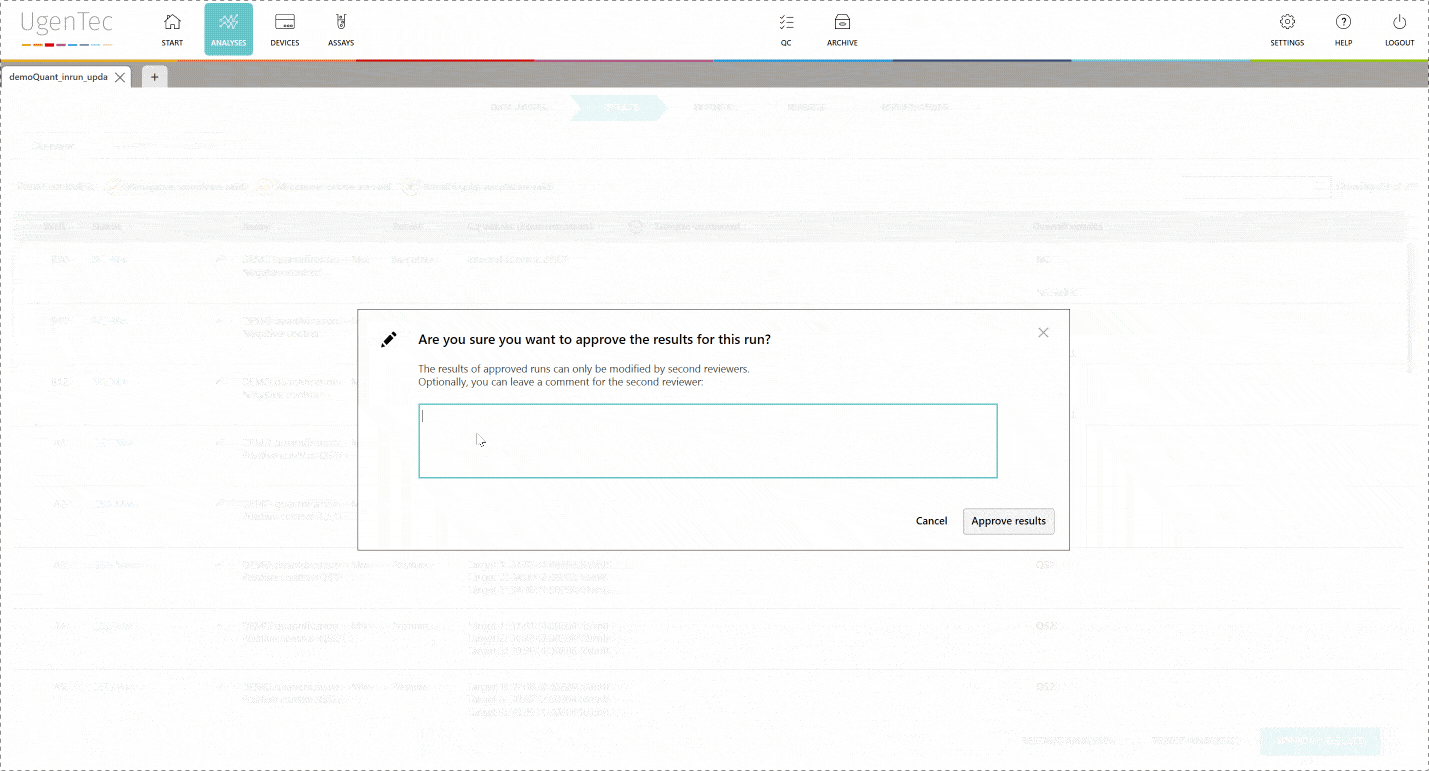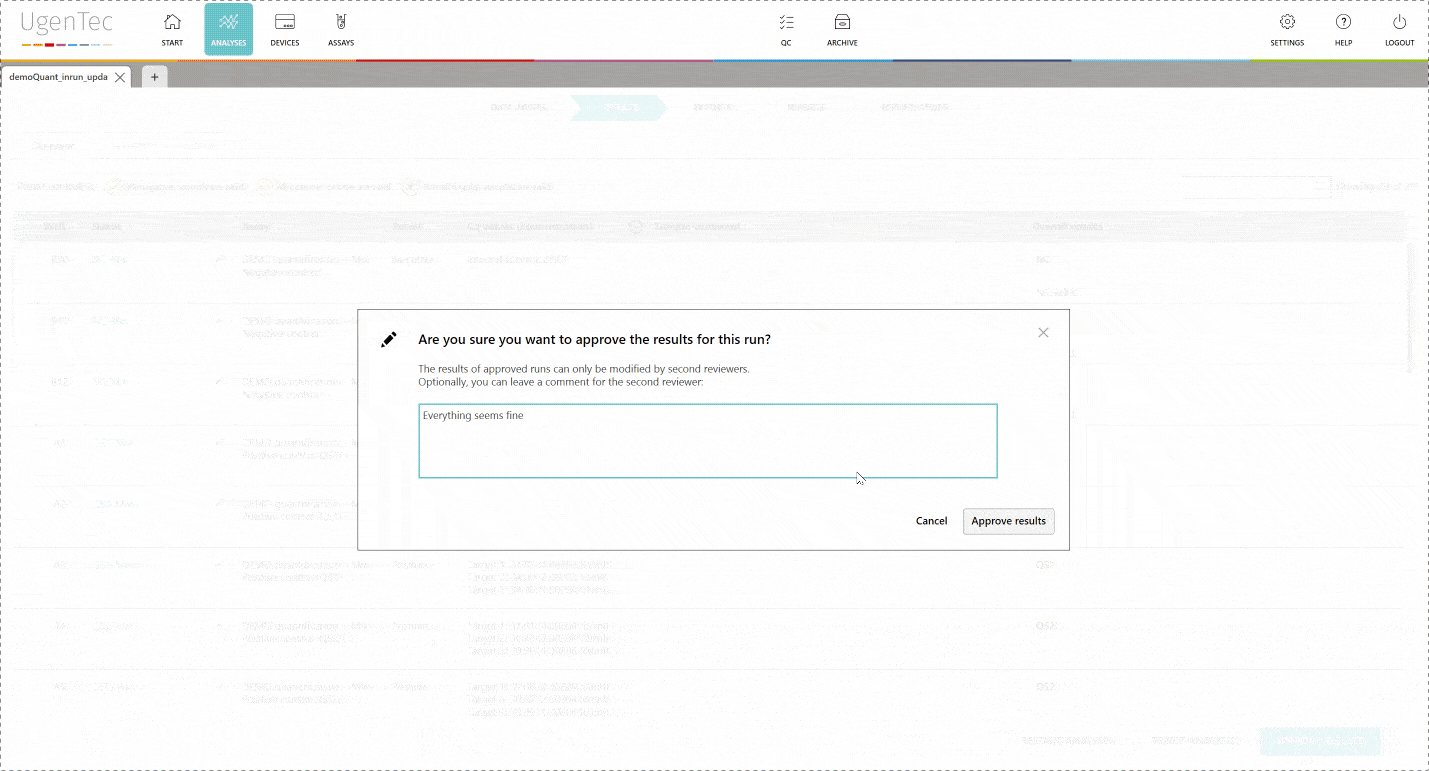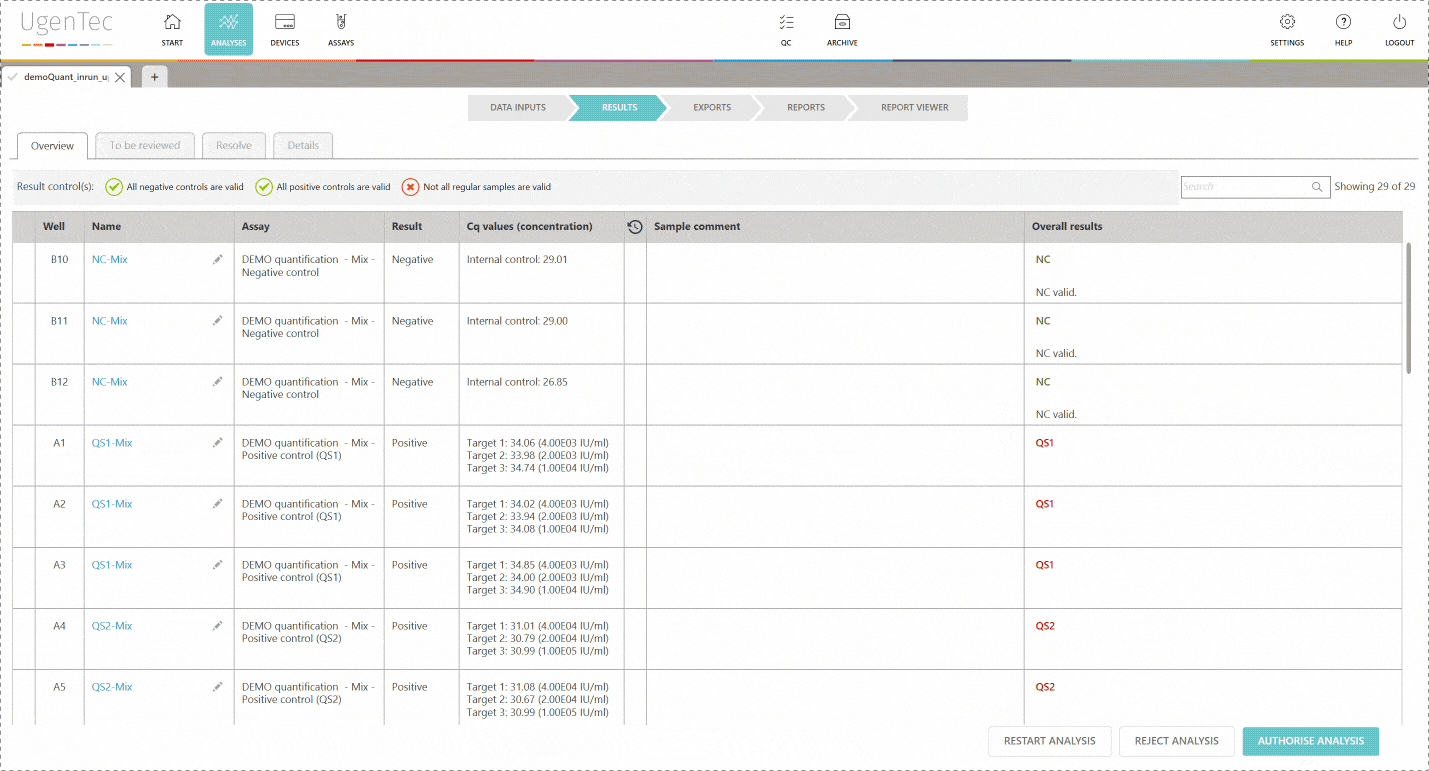
The second reviewer logs in and is immediately shown a list of "to be reviewed" runs. When he continues, he can authorize the results. Only from that point in time, it's possible to export them to a LIMS. PDF exports that aren't authorized will hold an unauthorized watermark.
New PCR platforms
Three more devices extend our list of compatible PCR devices:
- LightCycler 1.5
- Agilent Mx3005P
- Abbott m2000
Other, smaller changes
User actions and settings
A separate Settings module has been added. You can now distribute rights however you want to. For instance, setting up a particular user type to be unable to perform second validation.
It's also possible to toggle on and off QC tracking and the two-step validation mode.
Lot-dependent Cq cut-off values
For some assays, Cq cut-offs are determined by the manufacturer. For example, when you want to assess an internal control that's only valid when the Cq lies between 20-25. Decision trees in the software are now developed so that these Cq cut-offs- can be taken into account.
Sample rejection
Samples can now be rejected to ensure corrupted results never leave the diagnostic environment.
Assay plugin changes
If a plugin is updated, information already set up (lot, color compensation...) by you will not be lost. Rather it will be automatically transferred to the newest version of the plugin, once activated.
Other changes include:
- Decision trees are now also capable of processing replicates, based on a plugin that supports replicates and an identical sample name.
- We offer a better User Interface for single nucleotide polymorphism (SNP) plugins.
Plate templates
If a certain plate layout is used often and you don't want to use nametags to automate plate setup, you can now define a plate template and reuse it in any analysis to come.
Store data in preferred region
We are now capable of deploying FastFinder to a Microsoft Azure server center near you; this means we can now comply with more strict data privacy and data security regulation.
If your country upholds the CE-IVD mark as a regulatory approval and if local cloud storage is required by regulations, we can set up FastFinder in any of the regions specified in the link above.





