Founded in 2009 in Sydney, SpeeDx’ current IVD portfolio consists of two multiplex assays: ResistancePlus™ MG and PlexPCR™ HSV-1&2, VZV. The company is also developing a pipeline of multiplex assays including those for Respiratory viruses; HSV-1&2, VZV & Syphilis and STI panels.
The Australian diagnostics innovator is the first company to develop an assay for the detection of Mycoplasma genitalium and five mutations that are associated with azithromycin resistance. Combining antimicrobial screening with detection effectively allows medical doctors to choose the right treatment for patients, increasing efficiency and improving clinical outcomes.
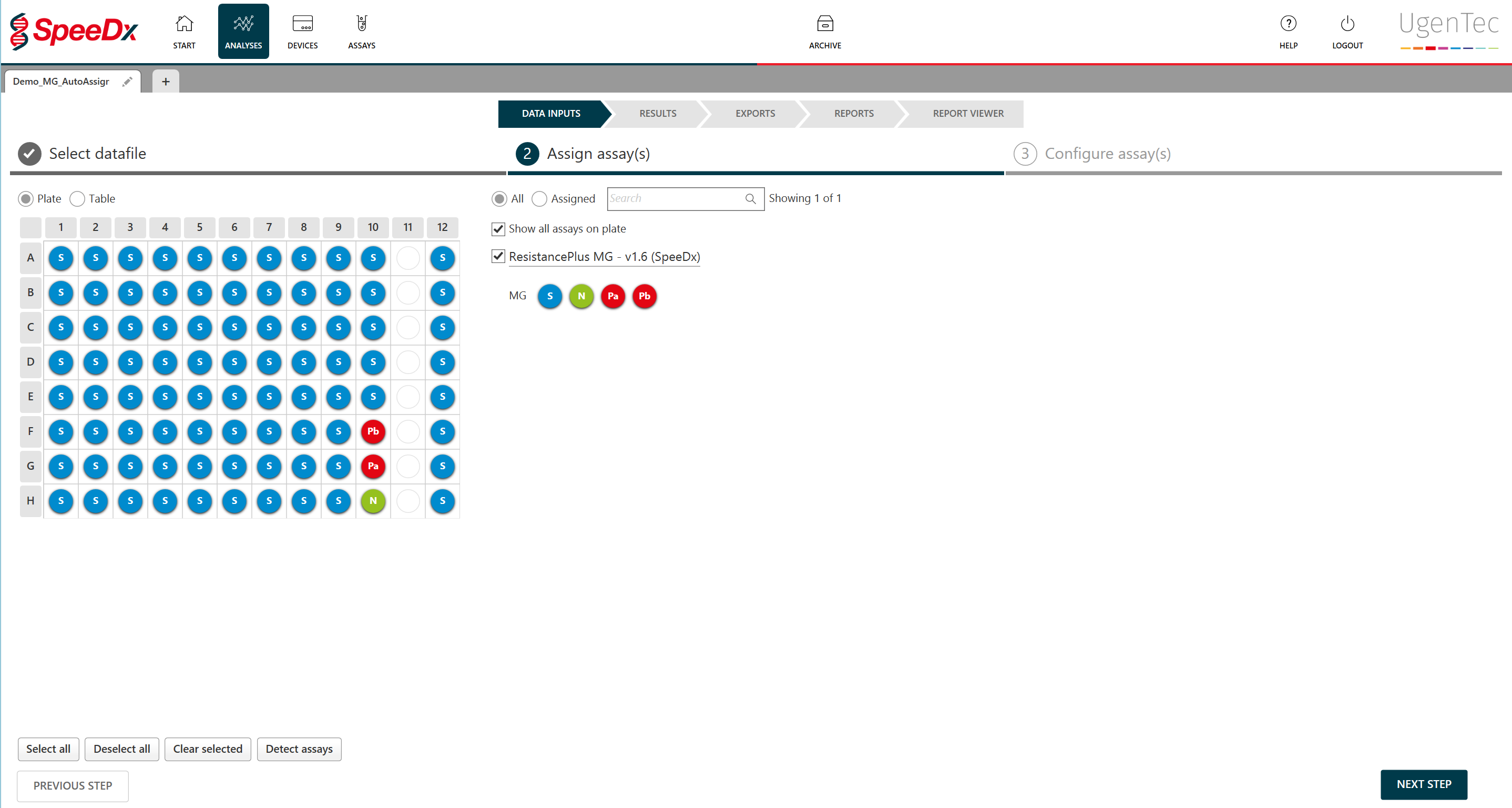
About the assay
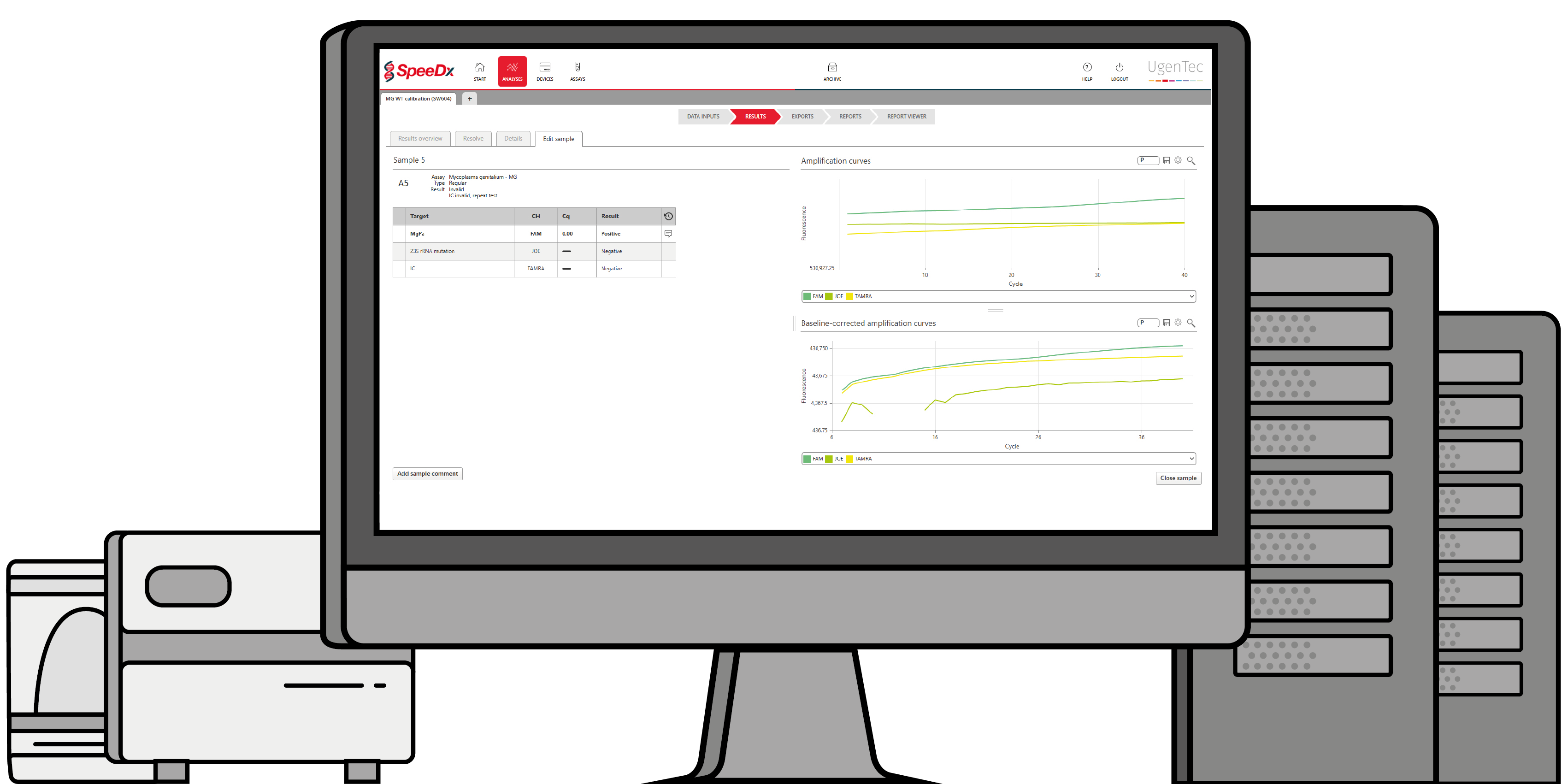
PlexPrime™ technology used in ResistancePlus™ MG offers greater specificity than other allele-specific primers, along with multiplexing capability to screen for multiple mutations in a single channel. As with most multiplex PCR tests, result interpretation can be challenging, especially with an advanced product like ResistancePlus™ MG.
Customers would question whether a result is real if the amplification looked inefficient or late. FastFinder removes the need for this eyes-on interpretation and reduces analysis time.
Litty Tan | Director of R&D
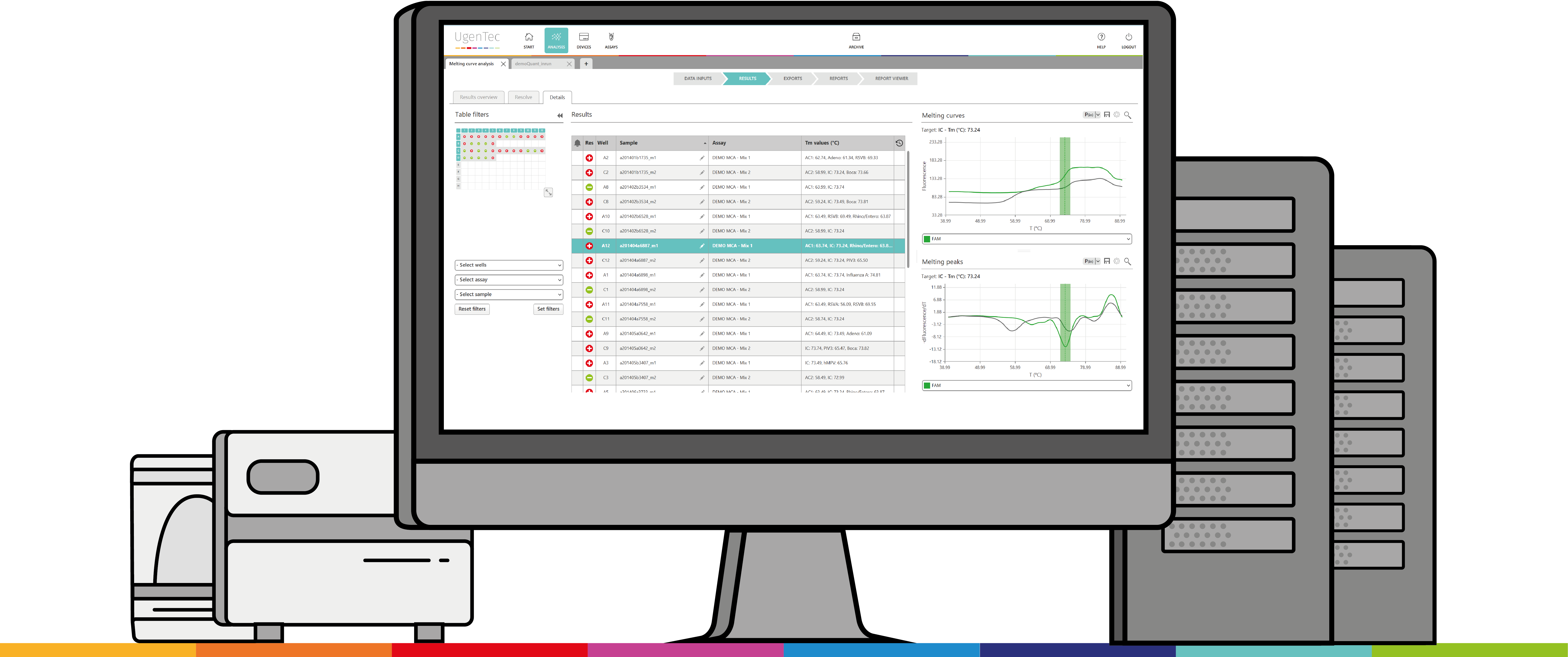
Turning a spreadsheet into a turnkey solution
SpeeDx end-users would use industrial software for the sample calling and copy-paste results into an MS Excel based analysis software to automate sample interpretation. However, the format wasn’t that user-friendly and still required some manual transferring of raw data.
Our Excel-based analysis software was limited in security and not as straightforward compared to an end-to-end solution like FastFinder
Samantha Walker | Quality Assurance Manager